Potentiometry
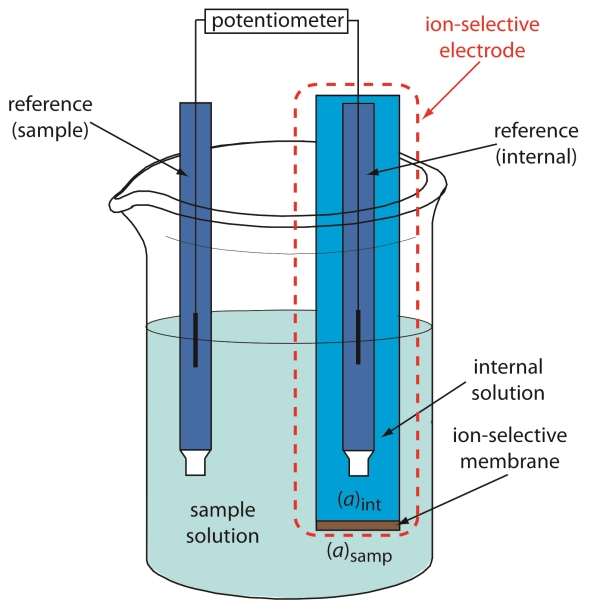
Potentiometric techniques are those techniques that measure the electrical potential difference in an electrochemical cell, and relate it to the activity of the ionic constituents in solution. To make a potentiometric measurement, a potentiometer, an indicator electrode, a reference electrode and an electrolyte solution containing the ion is required. Both electrodes are connected to a potentiometer and are immersed in the solution. The potential difference between the two electrodes is recorded and is referred to as the electromotive force (emf). The ideal indicator electrode not only needs to respond rapidly to changes in ion concentration, but it needs to respond in the presence of other ions found in the sample matrix. Therefore, an ideal indicator electrode is selective, reproducible, and has a useful lifetime. The most successful indicator electrode in potentiometry is the ion selective electrode (ISE) 2.
A liquid membrane ISE is made up of a thin film water-immiscible membrane containing an ion exchanger and an ion carrier. The ion of interest forms an ion pair with the exchanger. As a result, the ion pairs form stronger a bond than with any other ion likely to be present in the water16. The transport of ions across the membrane from a high concentration to a low one creates an electrical potential difference. This electrical difference is then transmitted to the meter, which converts the electric signal to a scale that is read in millivolts. The millivolts are then converted to mg/L of ion by plotting them from a standard curve.
Application: Nitrate Electrode Method
A nitrate ISE (used with a meter) is similar in function to a conductivity meter. A conductivity meter measures the capacity of water to conduct an electrical current and is a function of the types and quantities of dissolved substances in water. As concentrations of dissolved ions increase, specific conductance of the water increases 1. Hence, it is not possible to distinguish nitrate ions from other ions according to this method. For this reason, an ion selective electrode is used to determine nitrate concentrations in water. The accuracy of an ISE compares favorability with colorimetric methods for determining nitrates in water. Nitrate ISE can be affected by high concentrations of chloride and bicarbonate ions in the sample water. Fluctuating pH levels can also affect the reading by the meter 2 .
Nitrate ISE’s and meters are expensive compared to field kits that employ the cadmium reduction method. Field meter/probe combinations run between $700 and $1,200 including a long cable to connect the probe to the meter. If the program has a pH meter that displays readings in millivolts, it can be used with a nitrate ISE and no separate nitrate meter is needed. Results are read directly as milligrams per liter 3 .
Although nitrate electrodes and spectrophotometers can be used in the field, they have certain disadvantages. ISE devices are more fragile than the color comparators and are therefore more at risk of breaking in the field. They must be carefully maintained and must be calibrated before each sample run and between sampling sessions. This means that samples are best tested in the lab. Note that samples to be tested with a nitrate electrode should be at room temperature, whereas color comparators can be used in the field with samples at any temperature 4.
1 Radtke, D.B., Davis, J.V., and Wilde, F.D., 1998, Specific electrical conductance, in Wilde, F.D., and Radtke, D.B., eds., 1998, Field measurements, in National field manual for the collection of water-quality data: U.S. Geological Survey Techniques of Water Resources Investigations.
2 Fifield, F.W., and Haines, P.J. Environmental Analytical Chemistry. 2nd Ed. (2000). Malden, MA: Blackwell Science Ltd. Pg. 231.
3 Environmental Protection Agency. Nitrates. (6 March 2012) Web. Retrieved 30 Nov 2013 from http://water.epa.gov/type/rsl/monitoring/vms57.cfm.
4 APHA. 1992. Standard methods for the examination of water and wastewater. 18th ed. American Public Health Association, Washington, DC.